

 Location:Software Product > ZHQualiFly®
Location:Software Product > ZHQualiFly®
ZHQualiFly®excels in performance, is user-friendly, facilitates smoother multi-party communication, while improving the Quality Management System and reducing compliance risks.
Product Advantages:
Functional Features:
Autonomous configuration of multiple role permissions
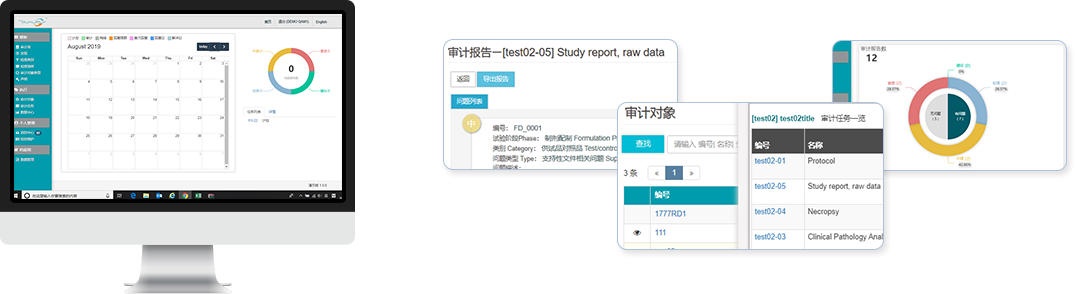
Supports users in autonomously configuring various role permissions, closely aligning with existing workflows. Account password strength and safety meet industry requirements.Integration of tasks and calendar
Task plans are displayed not only in list format but also bound to the calendar. End users can conveniently view their daily work tasks during their daily operations.Important events at a glance
The homepage displays important matters and significant findings to draw attention from multiple parties. Users can prioritize handling these events.Autonomous configuration of various templates
Users can set templates based on existing workflows and forms, including but not limited to problem types, inspection checklists, declaration content, etc.Audit/inspection reports, responses, and review processes are concise and efficient
Records every report, response, and review process. Supports uploading rectification evidence and appendices. Supports adding remarks and personnel updates.Various reminder functions
Supports task reminders, response reminders, rectification deadline reminders, etc. Reminders include internal system and external (email) notifications.Email binding
Supports binding of internal and external system emails. Users can receive the latest information from system-generated emails without logging into the system.Data statistics
Users can classify and analyze generated data to create reports and trend analysis charts.Electronic archiving
Supports electronic archiving of completed projects. Archived projects cannot be modified and have restricted read permissions.Electronic Record and Electronic Signature
Fully compliant with 21CFR Part11. Equipped with comprehensive audit trail functionality.